Advanced Bone Regeneration Technology Developed for Foot & Ankle Surgery
Advanced Bone Regeneration Technology Developed for Foot & Ankle Surgery
Advanced Bone Regeneration Technology Developed for Foot & Ankle Surgery
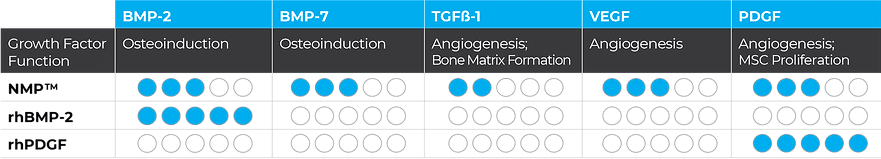
InduceXT is a human allograft which contains multiple growth factors naturally found in human bone. The bioimplant is created using the proprietary NMP® (Natural Matrix Protein) Process that unlocks growth factors, including BMP-2, BMP-7, TGF-ß1, VEGF, and PDGF, making them more bioavailable for bone regeneration.
InduceXT is a human allograft which contains multiple growth factors naturally found in human bone. The bioimplant is created using the proprietary NMP® (Natural Matrix Protein) Process that unlocks growth factors, including BMP-2, BMP-7, TGF-ß1, VEGF, and PDGF, making them more bioavailable for bone regeneration.
InduceXT is a human allograft which contains multiple growth factors naturally found in human bone. The bioimplant is created using the proprietary NMP® (Natural Matrix Protein) Process that unlocks growth factors, including BMP-2, BMP-7, TGF-ß1, VEGF, and PDGF, making them more bioavailable for bone regeneration.
NMP® TECHNOLOGY
NMP® TECHNOLOGY
NMP® TECHNOLOGY
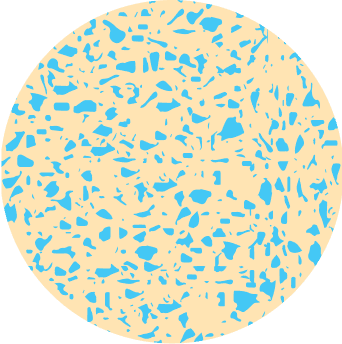
Demineralized Bone
Growth Factors Locked in Matrix with Limited Osteoinductivity
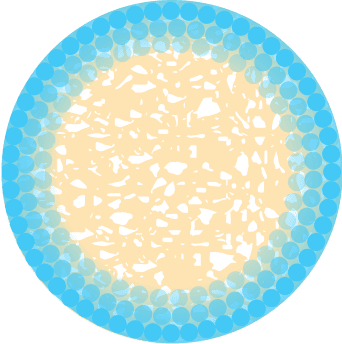
Processed NMP Bone
Growth Factors Unlocked from Matrix and Bioavailable
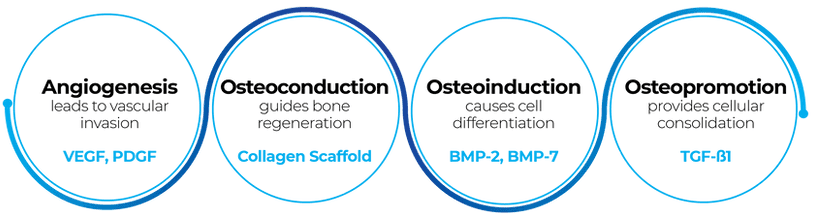
Osteoregeneration
Osteoregeneration
Osteoregeneration
Osteoregeneration




While bone grafts containing recombinant growth factors (rhBMP-2 and rhPDGF) release their growth factors in a burst, with over 90% released within 1 to 2 days, NMP® releases growth factors over a prolonged period, lasting into the bone remodeling phase.
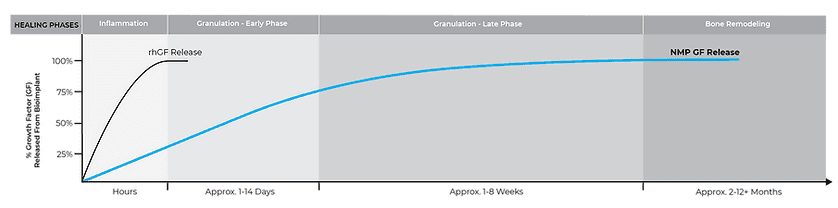



OSTEOINDUCTION
OSTEOINDUCTION
OSTEOINDUCTION
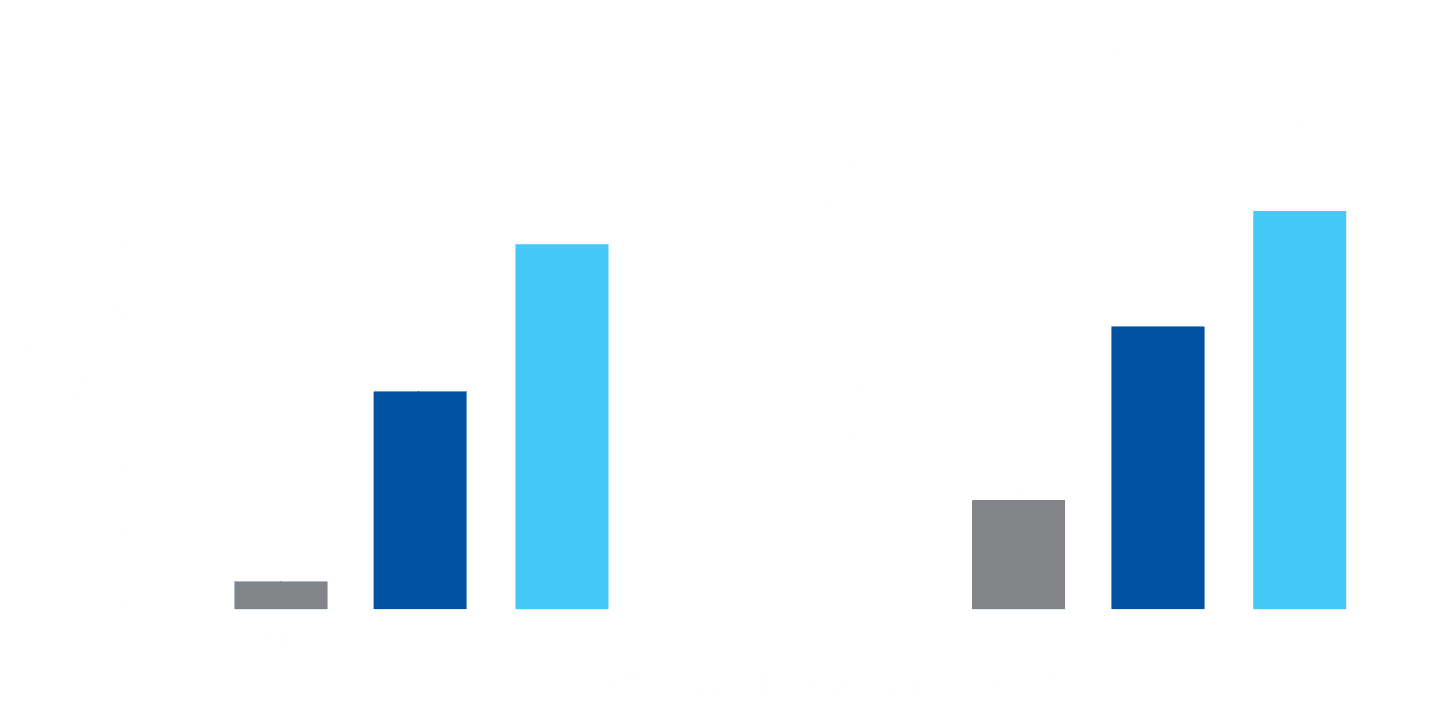
The NMP Bioimplant has been shown to form more bone of a better quality than rhBMP-2 in animal studies.
The NMP Bioimplant has been shown to form more bone of a better quality than rhBMP-2 in animal studies.
The NMP Bioimplant has been shown to form more bone of a better quality than rhBMP-2 in animal studies.



DBM

DBM

DBM

DBM
rhBMP-2

rhBMP-2

rhBMP-2

rhBMP-2
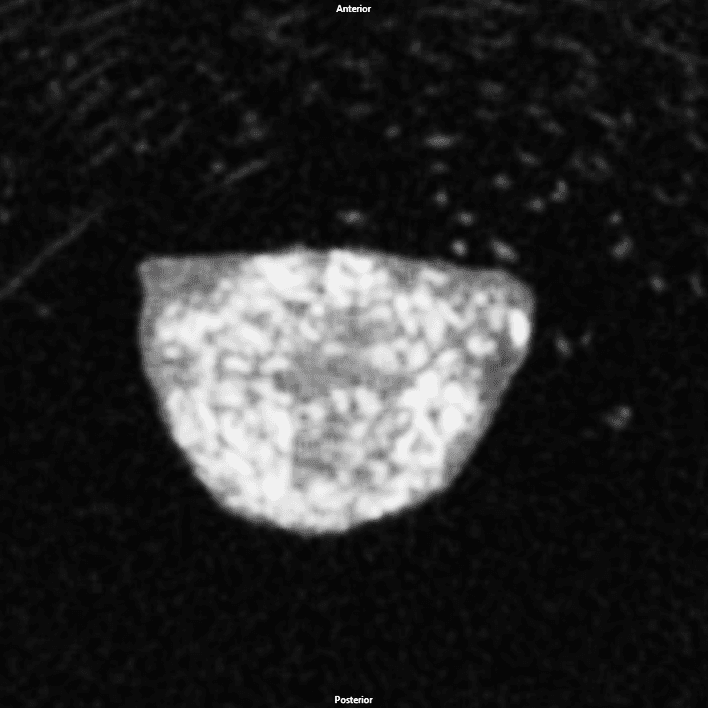
NMPᵀᴹ

NMPᵀᴹ

NMPᵀᴹ

NMPᵀᴹ
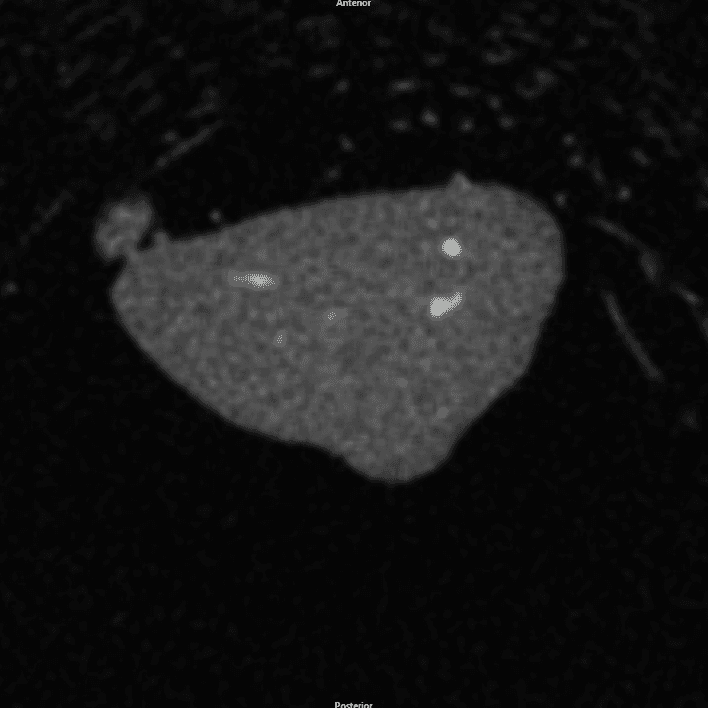
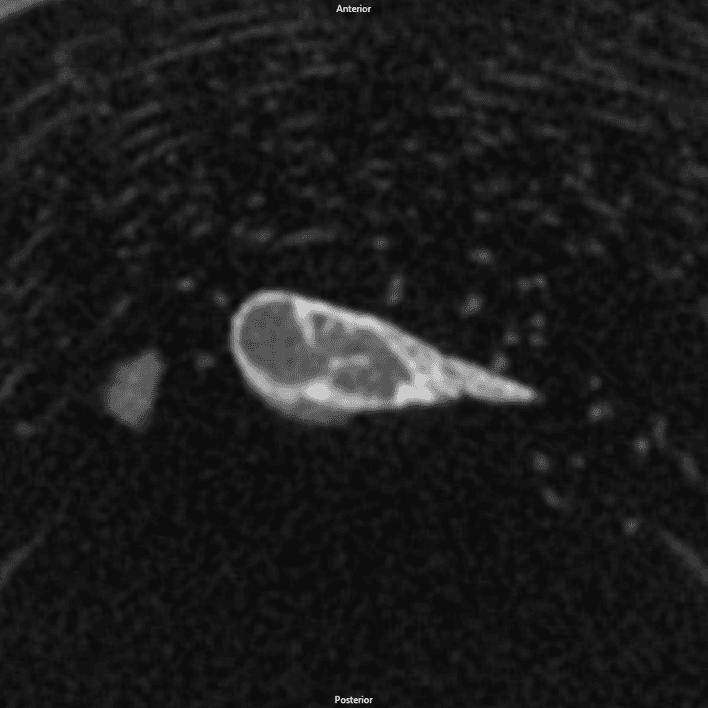
MicroCT images of DBM, rhBMP-2, and NMP implants 28 days post implantation
MicroCT images of DBM, rhBMP-2, and NMP implants 28 days post implantation
MicroCT images of DBM, rhBMP-2, and NMP implants 28 days post implantation
MicroCT images of DBM, rhBMP-2, and NMP implants 28 days post implantation
Gray mass is unmineralized tissue. White areas within the mass are mineralized bone, which was confirmed by histology.
Gray mass is unmineralized tissue. White areas within the mass are mineralized bone, which was confirmed by histology.
Gray mass is unmineralized tissue. White areas within the mass are mineralized bone, which was confirmed by histology.
Preparation and Handling
Preparation and Handling
Preparation and Handling
InduceXT is made up of long fibers, micro particulates, and mineralized cancellous bone.
InduceXT is made up of long fibers, micro particulates, and mineralized cancellous bone.
InduceXT is made up of long fibers, micro particulates, and mineralized cancellous bone.
Packable and able to withstand irrigation at the fusion site
Packable and able to withstand irrigation at the fusion site
Packable and able to withstand irrigation at the fusion site
Shelf-stable with a 5-year shelf life
Shelf-stable with a 5-year shelf life
Shelf-stable with a 5-year shelf life
Rehydrates with saline, bone marrow aspirate or blood
Rehydrates with saline, bone marrow aspirate or blood
Rehydrates with saline, bone marrow aspirate or blood
NMP InduceXT
NMP InduceXT
NMP InduceXT
NMP Fibers, NMP Micro Particulates, and mineralized cancellous bone
NMP Fibers, NMP Micro Particulates, and mineralized cancellous bone
NMP Fibers, NMP Micro Particulates, and mineralized cancellous bone
NMPXT10-0
NMPXT10-0
NMPXT10-0
InduceXT™
InduceXT™
InduceXT™
10.0cc
10.0cc
10.0cc
NMPXT3-0
NMPXT3-0
NMPXT3-0
InduceXT™
InduceXT™
InduceXT™
3.0cc
3.0cc
3.0cc
NMPXT1-5
NMPXT1-5
NMPXT1-5
InduceXT™
InduceXT™
InduceXT™
1.5cc
1.5cc
1.5cc





For Surgeons
For Surgeons
For Surgeons
InduceXT NMP® Bioimplant was developed exclusively for Foot & Ankle surgeons seeking and alternative to current market offerings.
InduceXT NMP® Bioimplant was developed exclusively for Foot & Ankle surgeons seeking and alternative to current market offerings.
InduceXT NMP® Bioimplant was developed exclusively for Foot & Ankle surgeons seeking and alternative to current market offerings.
Please contact us to be connected with a member of our team.
Please contact us to be connected with a member of our team.
Please contact us to be connected with a member of our team.
For Distributors
For Distributors
For Distributors
InduceXT NMP® Bioimplant is designed for independent distributors seeking a one-of-a-kind premium bone graft to compete with current market offerings.
InduceXT NMP® Bioimplant is designed for independent distributors seeking a one-of-a-kind premium bone graft to compete with current market offerings.
InduceXT NMP® Bioimplant is designed for independent distributors seeking a one-of-a-kind premium bone graft to compete with current market offerings.
Please contact us if you are interested in becoming an independent distributor.
Please contact us if you are interested in becoming an independent distributor.
Please contact us if you are interested in becoming an independent distributor.




Schedule A Call
Schedule A Call
Schedule A Call
Storage & Handling
NMP Bioimplants are freeze dried and sterile and should be stored at ambient temperature. To rehydrate and prepare product for use, cover allograft with whole blood, bone marrow aspirate (BMA), Lactated Ringers, normal saline, or other isotonic solution of choice. Please see Instructions for Use (IFU) included with each product for more information.
Tissue Procurement
We have partnered with tissue banks accredited by the American Association of Tissue Banks (AATB) to perform donor screening, tissue procurement procedures, and processing of human bone using our proprietary process to prepare our NMP™ products.
Please complete the Allograft Tissue Usage Form included with each product and return the form to the applicable tissue bank.
1. Kohen et al. Evaluation of the Natural Matrix Protein (NMP) bone allograft in vitro and in vivo. JBMR (2023) 38:S1 p342.
2. Data on file.
3. Peel SAF. The bone-forming potential of Natural Matrix Protein (NMP) bioimplants compared with cellular, peptide, and growth factor-enhanced bone graft substitutes. Presented at: The 38th Annual Meeting of the Canadian Biomaterials Society; Jun, 14-17 2023; Halifax, CA.
4. Induce Biologics NMP IFU.
Results from in vivo laboratory testing may not be predictive of clinical experience in humans. NMP products are intended for use as a bone void filler for filling voids and gaps in the skeletal system that are not intrinsic to the stability of the bony structure.
1. Kohen et al. Evaluation of the Natural Matrix Protein (NMP) bone allograft in vitro and in vivo. JBMR (2023) 38:S1 p342.
2. Data on file.
3. Peel SAF. The bone-forming potential of Natural Matrix Protein (NMP) bioimplants compared with cellular, peptide, and growth factor-enhanced bone graft substitutes. Presented at: The 38th Annual Meeting of the Canadian Biomaterials Society; Jun, 14-17 2023; Halifax, CA.
4. Induce Biologics NMP IFU.
Results from in vivo laboratory testing may not be predictive of clinical experience in humans. NMP products are intended for use as a bone void filler for filling voids and gaps in the skeletal system that are not intrinsic to the stability of the bony structure.
1. Kohen et al. Evaluation of the Natural Matrix Protein (NMP) bone allograft in vitro and in vivo. JBMR (2023) 38:S1 p342.
2. Data on file.
3. Peel SAF. The bone-forming potential of Natural Matrix Protein (NMP) bioimplants compared with cellular, peptide, and growth factor-enhanced bone graft substitutes. Presented at: The 38th Annual Meeting of the Canadian Biomaterials Society; Jun, 14-17 2023; Halifax, CA.
4. Induce Biologics NMP IFU.
Results from in vivo laboratory testing may not be predictive of clinical experience in humans. NMP products are intended for use as a bone void filler for filling voids and gaps in the skeletal system that are not intrinsic to the stability of the bony structure.
Contact us
Additional Links
Subscribe now
Get the latest from InduceXT
© 2024 Induce Biologics
Contact us
Additional Links
Subscribe now
Get the latest from InduceXT
© 2024 Induce Biologics
Contact us
Additional Links
Subscribe now
Get the latest from InduceXT
© 2024 Induce Biologics
Contact us
Additional Links
Subscribe now
Get the latest from InduceXT
© 2024 Induce Biologics



